Hydrogen is a versatile energy carrier that can be used to power nearly every end-use energy need. Hydrogen is the simplest and most common element in the universe. It is a colorless, odorless, and tasteless gas that has the highest energy content per unit of weight of any known fuel. Hydrogen is very chemically active and rarely stands alone as an element. It usually exists in combination with other elements, such as oxygen in water, carbon in methane, and in trace elements as organic compounds. Hydrogen therefore must be broken from its bonds with other elements in order to be used as a fuel. There are numerous processes that can be used to break these bonds.
Hydrogen is a source of renewable, clean energy, which can be used in fuel cells to generate electricity. Hydrogen can also be used to provide power in another way through a process known as combustion. Hydrogen can provide power in two ways: hydrogen fuel cells can be used to create electricity or hydrogen combustion engines can be used to power vehicles. Combustion engines have been designed to burn hydrogen to power vehicles, instead of burning the non-renewable fuel petrol or diesel. Traditional vehicles are powered by a mix of petrol or diesel and air that is ignited in a combustion engine. This releases energy that causes pistons in the engine to move, which in turn produces power to make the wheels go round. However, when petrol and diesel are burnt they produce the greenhouse gas carbon dioxide (CO2) which contributes to climate change.Hydrogen powered vehicles have a type of combustion engine specifically designed to burn hydrogen. When hydrogen is burnt in the presence of air, energy is released that powers the car, in a similar way to petrol or diesel engines. However, hydrogen has an advantage over petrol or diesel. When hydrogen is burnt it only produces pure water, whereas traditional engines give off CO2 and other pollutants.
Vehicles that run on hydrogen are already in existence although they are not yet in general production. One of the main problems associated with hydrogen powered vehicles is the availability of fuelling stations. However, there have been several hydrogen fuelling stations operating in Europe since 1999 and more are opening. Hydrogen is known as clean energy as it does not produce CO2. The benefits of liquid hydrogen make it an important source of fuel for the future to use for example in power plant as a feedstock.
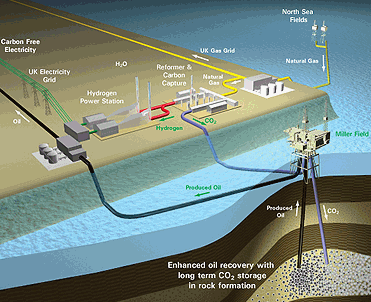
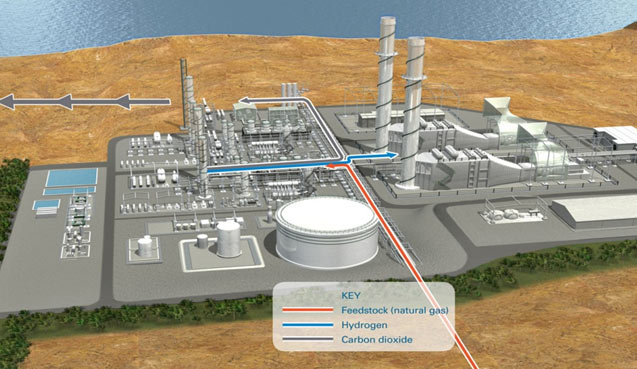
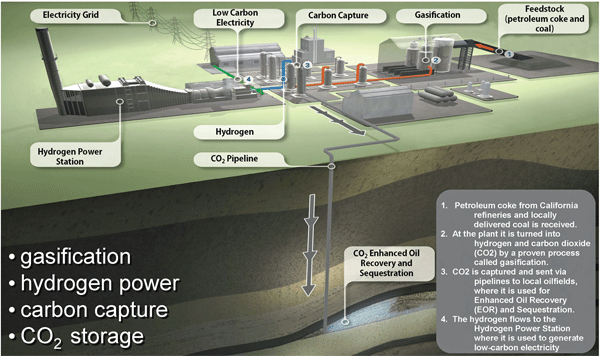
Hydrogen and pre-combustion CO2 capture systemsA pre-combustion capture process typically comprises a first stage of reaction producing a mixture of hydrogen and carbon monoxide (syngas) from a primary fuel. The two main routes are to add steam (reaction 1), in which case the process is called ‘steam reforming’, or oxygen (reaction 2) to the primary fuel. In the latter case, the process is often called ‘partial oxidation’ when applied to gaseous and liquid fuels and ‘gasification’ when applied to a solid fuel, but the principles are the same.
Steam reforming: CH4 + H2O → CO + 3 H2 (1)
Partial oxidation: CH4+1/2O2→CO+2H2 (2)
This is followed by the ‘shift’ reaction to convert CO to CO2 by the addition of steam (reaction 3): Water Gas Shift Reaction: CO+H2O⇔CO2+H2 (3)
Finally, the CO2 is removed from the CO2/H2 mixture. The separated CO2 is then available for storage. It is possible to imagine two applications of pre-combustion capture. The first is in producing a fuel (hydrogen) that is essentially carbon-free. Although the product H2 does not need to be absolutely pure and may contain low levels of methane, CO or CO2, the lower the level of carbon-containing compounds, the greater the reduction in CO2 emissions. The H2 fuel may also contain inert diluents, such as nitrogen (when air is typically used for partial oxidation), depending on the production process and can be fired in a range of heaters, boilers, gas turbines or fuel cells specially as fuel for power plants. Secondly, pre-combustion capture can be used to reduce the carbon content of fuels, with the excess carbon (usually removed as CO2) being made available for storage.
Today’s emerging hydrogen energy industry is eager to develop hydrogen fuel infrastructure technology that can be used to generate power for stationary power plants, transportation, and portable power applications. Much work needs to be done to reach this goal, but a foundation for future efforts has been established by these various technology sectors. Areas of interest in turbine development include fuel flexible turbines, turbines for large-scale fuel cell hybrid systems, “sequestration-ready” turbines that use hydrogen or burn natural gas in oxygen, and other innovative turbine concepts. Building upon existing platforms such as Advanced Turbine Systems, turbine technology will be extended by applying expertise in heat transfer, fluid 13 dynamics, and advanced materials. Performance targets are 60% efficiency (higher heating value) for systems based on coal with emissions of NOx and SO2 at single digit levels. Vision 21 turbines will operate at extremely high temperatures (3000oF) and will be integrated with fuel gas cleaning and air separation systems. System dynamics will be a major factor in designing turbines for fuel cell hybrid systems. Natural gas/oxygen and hydrogen turbines must overcome challenges related to mixing and ultra-high temperature operation.
Rahbord Energy Alborz Scope on Hydrogen EnergyIn order to improve air quality and energy consumption, we need to focus on using hydrogen as fuel for industries and power plants. In this direction, Rahbord energy corporation has extensive studies on application of hydrogen as fuel to utilize in direct combustion. Our projects are underway to develop absorption of pre-combustion hydrogen. Besides, our research group has many efforts in modifying energy performance and sequestrating CO2 by performing of suitable solution to achieve sustainable development of energy and clean environment.